This report describes the genetic characteristics of the complete genomes of 27 European HPAI H5N8, H5N5 and H5N1 viruses recently identified in Belgium, Croatia, Denmark, the United Kingdom, Germany, Italy, the Netherlands, Poland and Sweden. Analyses are based on sequences produced by the EURL or deposited in GISAID by Member States (available on December 7, 2020).
Phylogenetic analysis
The phylogenetic analysis of the HA gene reveals that all the European HPAI H5 viruses form a single genetic group within clade 2.3.4.4B, and cluster together with HPAI H5 viruses identified in Kazakhstan and in the Russian Federation starting from July 2020 (Figure 1). The H5N8 strain collected in Iraq in May 2020 and the H5N8 strains circulating in Egypt since 2017 are the most related viruses, suggesting that North Africa and South-West Asia might be the areas where the progenitors of the recent HPAI European viruses have circulated in the last few years; however, more genetic data from these regions are necessary to confirm this observation.
Reassortant genotypes
The characterization of the complete genome revealed that the European HPAI H5 viruses show distinct gene constellations, likely due to the occurrence of multiple reassortment events with LPAI viruses circulating in wild birds in Eurasia and Africa. According to the different gene composition of each analysed virus, we identified 5 distinct genotypes, consisting of one H5N8, one H5N1 and three H5N5 subtype viruses (Figure 2, obtained from the Scientific report: Avian influenza overview August – December 2020, in the EFSA Journal 2020). Four of these genotypes have been detected in Europe (namely i, ii, iv, v).
- H5N8 viruses have been identified and sequenced in Belgium, Croatia, Denmark, the United Kingdom, Germany, Italy, the Netherlands, Poland and Sweden. The H5N8 viruses cluster together and with H5N8 strains identified in Iraq and Egypt for all the gene segments.
- H5N1 viruses have been identified and sequenced in the Netherlands and Italy. H5N1 viruses result from multiple reassortment events with LPAI viruses circulating in wild birds in Eurasia, from which they have acquired six (PB2, PB1, PA, NP, NA and NS) out of eight (6/8) gene segments. As for the HA and M gene segments, they cluster with the HPAI H5N8 European viruses.
Three genotypes have been identified among the H5N5 viruses.
- The Russian H5N5 viruses show the same genetic constellation of the H5N8 viruses except for the NA gene, which has been acquired from Russian LPAI viruses circulating in wild birds.
- The German and Belgian H5N5 viruses cluster with the HPAI H5N8 European viruses for the PB2, PB1, HA, NP, M and NS genes, and with Russian LPAI viruses circulating in wild birds for the NA and PA gene.
- The Danish H5N5 virus clusters with the HPAI H5N8 European viruses for the HA, M, PB1, NS genes; with the HPAI H5N5 virus from Germany and Belgium for the NA and PA genes; and with LPAI viruses circulating in wild birds in Eurasia and Africa for the PB2 and NP genes.
Spatial analysis
To investigate the dynamics of virus incursions and spread throughout Europe, we performed a discrete phylogeographic analysis based on the HA gene segment of the European H5Nx viruses. This investigation revealed that new viral introductions from Central Asia to Europe have occurred during the autumn wild bird migrations. We identified an intense viral movement among northern European countries (the Netherlands, Germany, Belgium, and Denmark) and a viral spread from North to South Europe (Figure 3). A more detailed phylogeographic reconstruction in continuous space supports these findings, further suggesting the occurrence of at least three different viral introductions from north to the southern European countries (Italy and Croatia) (video).
Acknowledgments
We gratefully acknowledge the authors, originating and submitting laboratories of the sequences from GISAID’s EpiFlu™ Database on which these analyses are based.
We wish to thank the following Member State representatives who shared sequence data: Mieke Steensels (Belgium), Vladimir Savic (Croatia), Charlotte Hjulsager (Denmark), Timm Harder (Germany), Krzysztof Śmietanka (Poland), Nancy Beerens (The Netherlands), Siamak Zohari (Sweden) as well as Ian Brown from APHA (United Kingdom) and Ilya Chvala from Federal Center for Animal Health (FGBI ‘ARRIAH’, Russia).
References
EFSA (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control), EURL (European Reference Laboratory for Avian Influenza), Adlhoch C, Fusaro A, Gonzales JL, Kuiken T, Marangon S, Niqueux É, Staubach C, Terregino C and Baldinelli F, 2020. Scientific report: Avian influenza overview August – December 2020. EFSA Journal 2020;18(12):6379, 57 pp. doi:10.2903/j.efsa.2020.6379.
Figures & Video
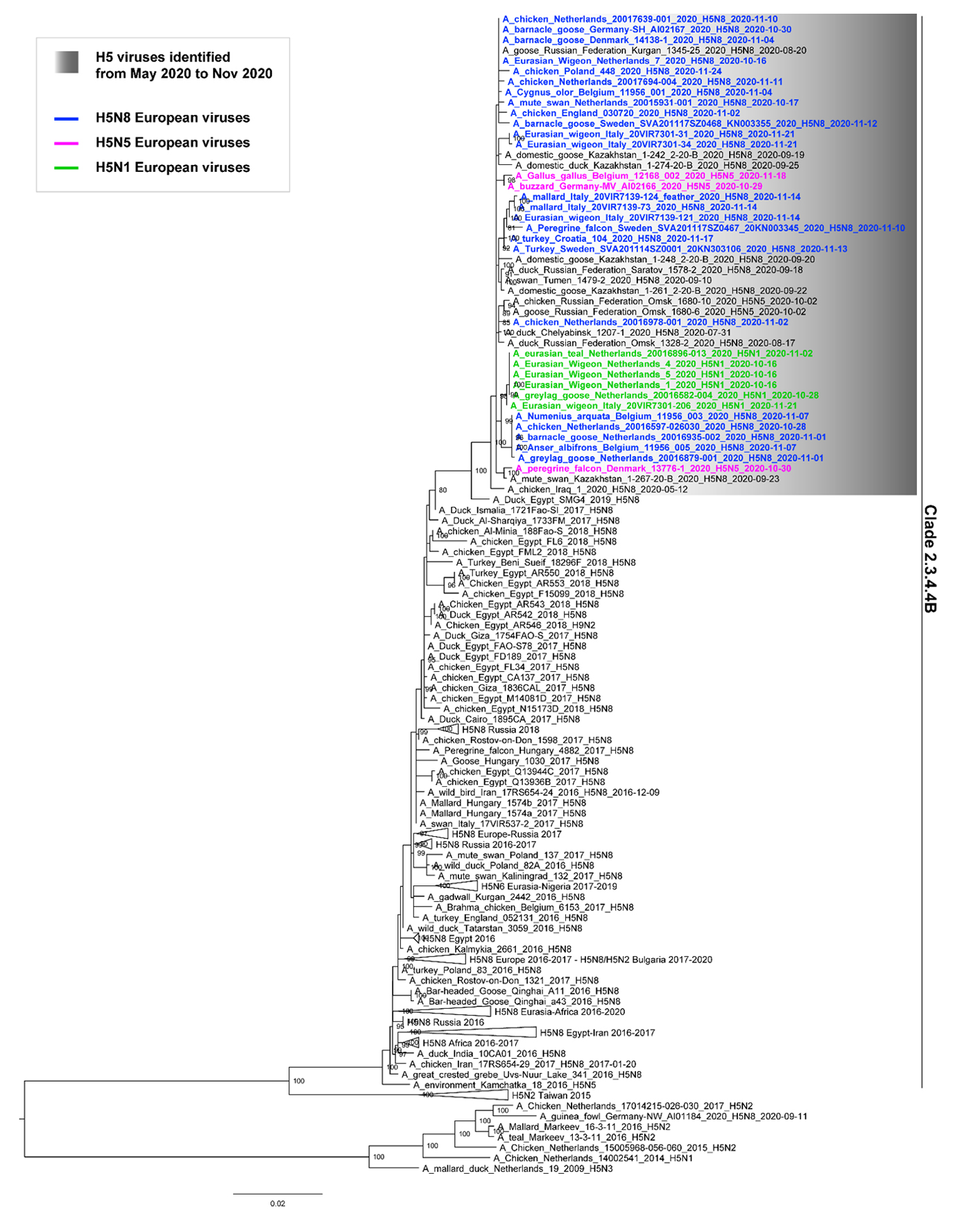
Figure 1. Maximum Likelihood phylogenetic tree of the HA gene (clade 2.3.4.4B). The grey box shows the H5 viruses identified between May and November 2020. The H5N8 European viruses are marked in blue; the H5N5 European viruses are marked in pink; the European H5N1 viruses are marked in green. Bootstrap supports higher than 80 are indicated next to the nodes.

Figure 2. HPAI A(H5) genotypes identified in Eurasia since May 2020 and their geographic detection based on the available sequences. Blue bars: gene segments originating from Eurasian/African HPAI H5N8 of the epidemic wave started in 2016; green bars: gene segments closely related to LPAI viruses circulating in wild birds in Eurasia; pink bars: gene segments closely related to LPAI viruses identified in wild birds in Russia; yellow bars: gene segments closely related to LPAI viruses identified in wild birds in Asia and Africa. This figure has been kindly provided by the Scientific report: Avian influenza overview August – December 2020, EFSA Journal 2020 (doi:10.2903/j.efsa.2020.6379).

Figure 3. The map shows statistically supported non-zero rates for the H5 gene segment of HPAI H5Nx viruses identified in Eurasia starting from May 2020. The thickness of the dashed lines is proportional to the relative strength by which rates are supported: very strong (BF > 150), strong (20 < BF < 150) and positive (10 < BF < 20). Each country is labelled by the same colour used in the annotated Maximum Clade Credibility (MCC) Tree of the HA gene (top left), which shows evolutionary relationships among H5 viruses from Europe, Central Asia, Egypt and Iraq.
Video. Spatiotemporal dispersal patterns of the HPAI H5Nx viruses collected in Europe, Russia and Kazakhstan between 1st July and 24th November 2020, inferred using continuous phylogeographic analysis. The black lines and the dots represent part of the branches and the nodes of the Maximum Clade Credibility (MCC) Tree of the HA gene, obtained from the continuous phylogeographic analysis. Contours represent statistical uncertainty of the estimated locations at the internal nodes (95% credible contours based on kernel density estimates).